BOTOX®
(OnobotulinumtoxinA) BOTOX® (the Brand name) is the classic neuromodulator on the market and has been in use for almost 20 years. It was originally approved for use in 2002 and remains the most popular type of neuromodulator. BOTOX® contains certain protective proteins that some people may develop antibodies against, which will lessen the effect over time. This is what people call “BOTOX® resistance” and why it is sometimes recommended to alternate between using different products. BOTOX® is produced by Allergan and is manufactured with the strictest safety protocols at their facility in Ireland. You can read more about the manufacturing process in my blog post. There is also a therapeutic version of BOTOX® that can be used to treat medical conditions like migraines, excessive sweating, and eye spasms.
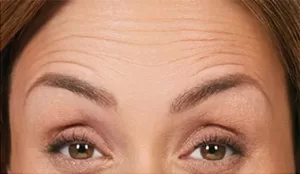
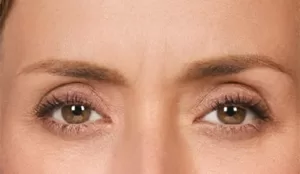
Actual patient. Results may vary. By prescription only. Photos taken at maximum eyebrow elevation before and 30 days after treatment with BOTOX® Cosmetic. In 2 clinical studies of healthy adults, 61% and 46% had a ≥ 2-grade improvement at day 30.1,*
*Side effects associated with the injection include localized pain, infection, inflammation, tenderness, swelling, redness, and/or bleeding/bruising.1
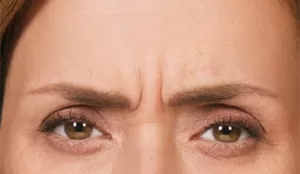
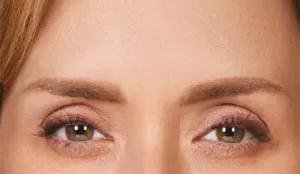
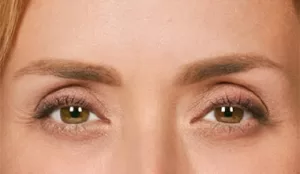
Moderate to severe frown lines
Actual patient. Results may vary. By prescription only.
Photos taken at maximum frown before and after treatment with BOTOX® Cosmetic at day 30, day 90, and day 120. In clinical studies,
physicians assessed 80% of adults had significant improvement at day 30; 48% of adults had significant improvement at day 90; and
25% of adults had significant improvement at day 120; in the same studies, 89% of adults who were treated saw at least moderate
improvement at day 30, 63% saw at least moderate improvement at day 90, 39% saw at least moderate improvement at day 120.1,*
* Side effects associated with the injection include localized pain, infection, inflammation, tenderness, swelling, redness, and/or bleeding/bruising.1
Download: BOTOX® Cosmetic Important Safety Information.